Describe a System Buffered With Carbonic Acid
The carbonic anhydrase is then split into CO 2 and H 2 O. Carbonic acid can change the effective concentration of a basic eluent including solutions of sodium hydroxide bicarbonate and carbonate.

Solved 5 Carbonic Acid Bicarbonate Buffer One Of The Most Chegg Com
The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers.
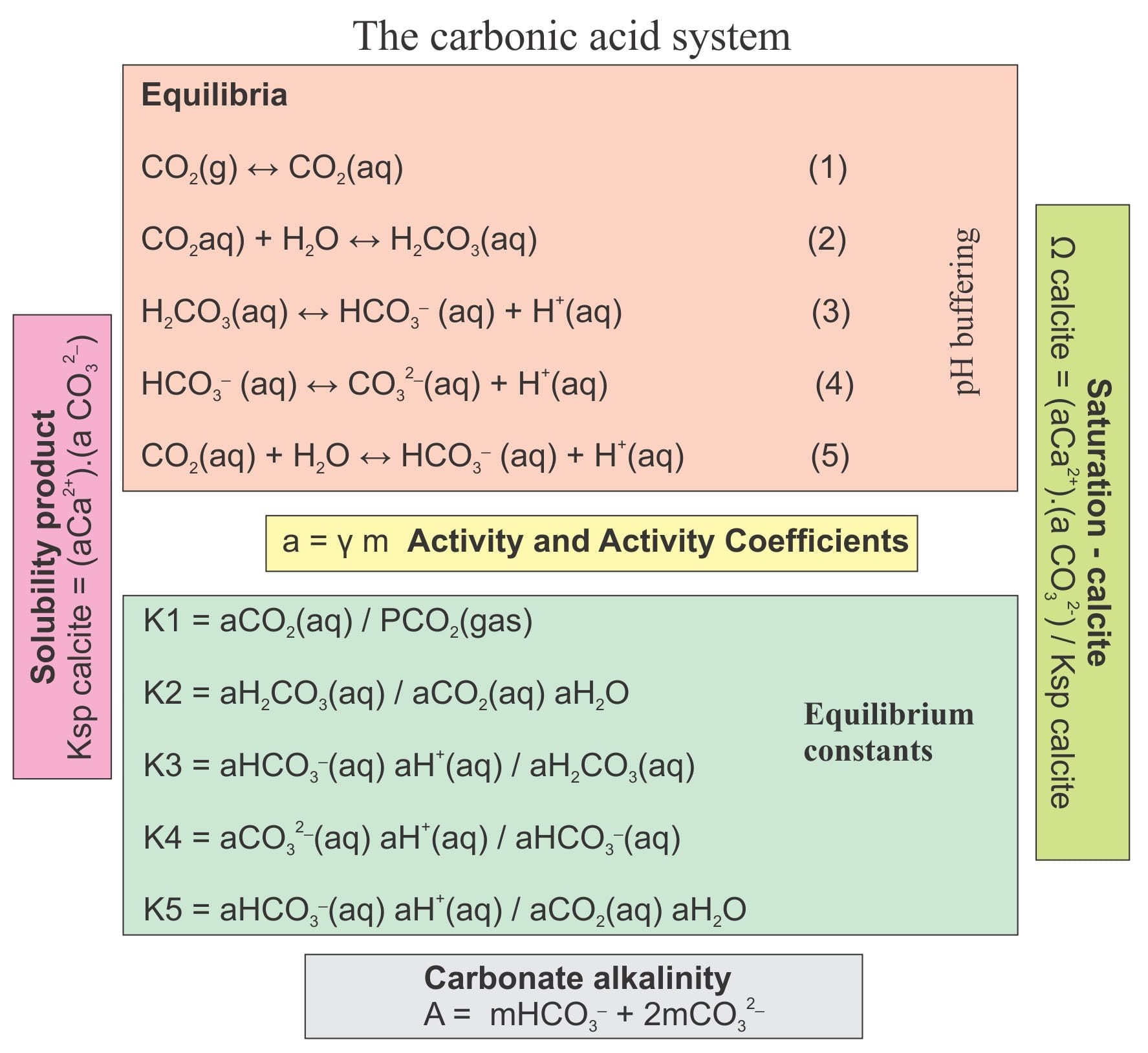
. The maintenance of acidbase homeostasis is a major function of the body accomplished by intracellular and extracellular buffering mechanisms exhalation of carbon dioxide CO 2 through the respiratory system and acid excretion by the kidneys In healthy individuals ingesting a typical Western diet metabolism produces acid or alkali components. CO 2 diffuses into the cell where CO 2 is rehydrated to carbonic acid. This gives rise to bicarbonate ion that exits from the cell through the basolateral membrane into the interstitium via a 3HCO 3 Na NBCe1 symporter while H is secreted again into the lumen.
The salt of the strong acid. In the carbonic acidbicarbonate buffer system strong acids are buffered by a. Lactic acid is produced in our muscles when we exercise.
It was evident as early as 1922 that factors other than P co 2 HCO 3 pK 1 and S influence plasma pH. As the lactic acid enters the bloodstream it is neutralized by the ceHCO3- ion producing H 2 CO 3. When sodium bicarbonate NaHCO 3 comes into contact with a strong acid such as HCl carbonic acid H 2 CO 3 which is a weak acid and NaCl.
They are found in the highest density in oily areas such as the face scalp and upper trunk. Academiaedu is a platform for academics to share research papers. This is not the case in seawater.
6 This finding shows that the Henderson-Hasselbalch equation cannot be accurately applied to mammalian blood cooled in. In distilled water the addition of HCl leads to an increase of H and Cl in solution in a ratio 11. Usually degassed water is used to prepare eluents and efforts should be made to keep exposure of eluent to air to a.
Red blood cells RBCs also referred to as red cells red blood corpuscles in humans or other animals not having nucleus in red blood cells haematids erythroid cells or erythrocytes from Greek erythros for red and kytos for hollow vessel with -cyte translated as cell in modern usage are the most common type of blood cell and the vertebrates principal means of. Describe a capillary wall. The bicarbonate is regulated in the blood by sodium as are the phosphate ions.
CO 2 in the blood readily reacts with water to form carbonic acid and the levels of CO 2 and carbonic acid in the blood are in equilibrium. They can cause skin disease by overgrowth descending into hair follicles or through inflammation. An enzyme then accelerates the breakdown of the excess carbonic acid to carbon dioxide and water which can be eliminated by breathing.
Henderson was broadly knowledgeable. 16 In 1925 pK 1 and therefore pH was shown to be influenced by ionic strength μ 17 and temperature 18 the ΔpK 1 K 1 K 1. Read Doc 117 b p s xi chemistry iit jee advanced study package 2014 15 by SDharmaraj on Issuu and browse thousands of other publications on our p.
Where Do you Find Malassezia. He wrote an equation in 1908 to describe the carbonic acid-carbonate buffer system in blood. Academiaedu is a platform for academics to share research papers.
In addition to his important research on the physiology of blood he also wrote on the adaptations of organisms and their fit with their environments on sociology and on university education. Malassezia is most likely to be found in oily parts of the skin where they sustain themselves by ingesting the fatty acids present in normal sebum. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid Figure 2642.
For example the addition of 1 µmol kg1 HCl to. Of a strong acid such as hydrochloric acid naturally added to the ocean by volcanism is strongly buffered by the seawater carbonate system.

What Is The Carbonic Acid Bicarbonate Buffer System Brainly In

Solved 47 Buffers Help To Protect Contents Of Cells From Excess Acid Base The Primary Buffer System That Controls The Ph Of The Blood Is The Carbonic Acid Bicarbonate Buffer System The Principal Organs

Le Chatelier S Principle Grade 12u Chemistry Systems And Equilibrium

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
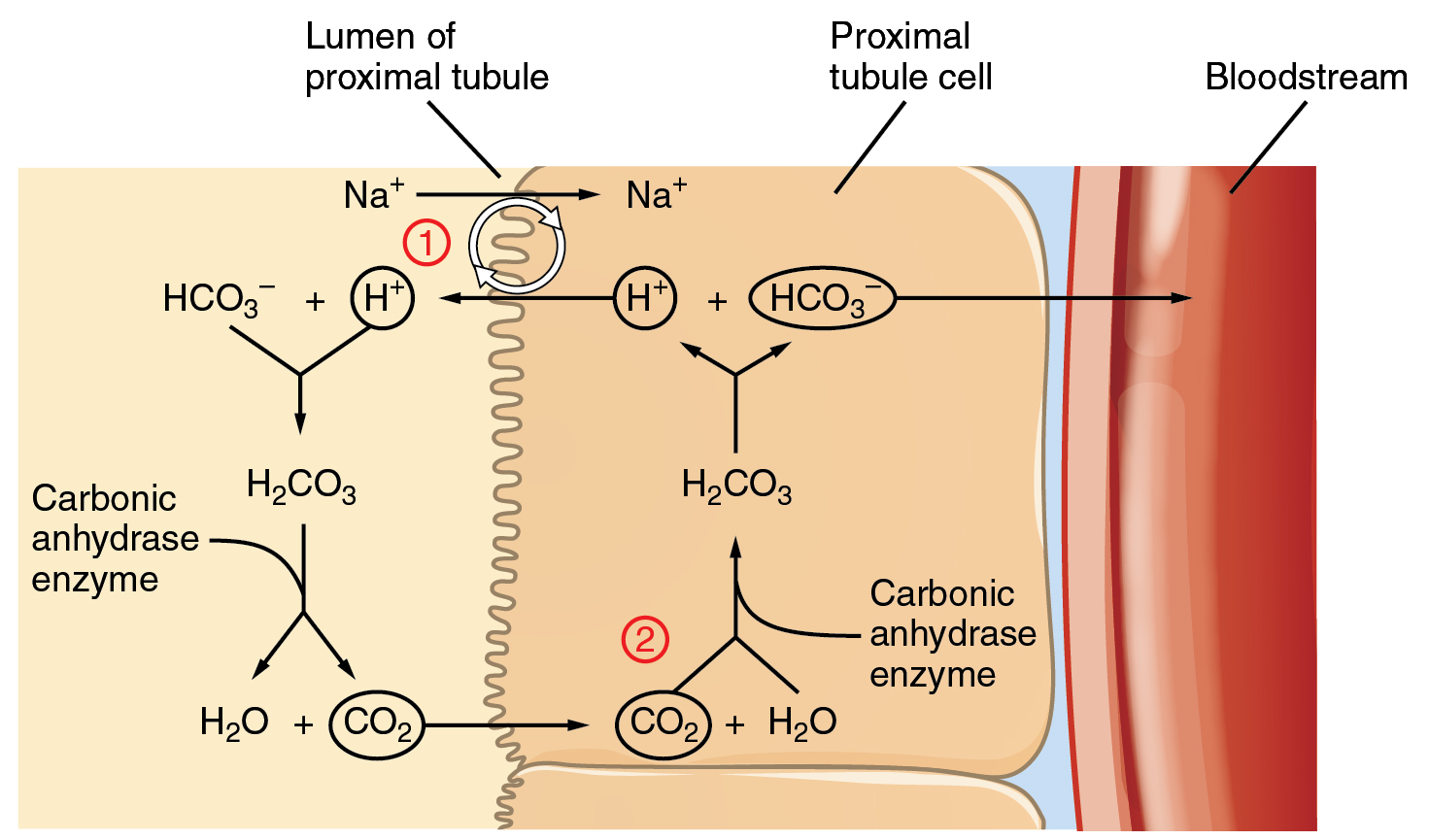
Acid Base Balance Anatomy And Physiology

Solved Answer The Following Question Regarding A Carbonic Chegg Com

Solved Blood Is Buffered By The Carbonic Acid Bicarbonate Buffer System Hzco3 Hzo Hco3 Haot If A Relatively Small Amount Of A Strong Acid Were Added To This Buffer Which Component Of The

Understanding The Carbonic Acid Bicarbonate Buffer In Hydroponics Science In Hydroponics

Bicarbonate Buffer System Flashcards Quizlet

Solved Human Blood Is Slightly Basic In Nature With Normal Ph Range Of 7 35 7 45 The Blood Ph Is Maintained By The Carbonic Acid Bicarbonate Buffer System Shown By The Following Equilibrium Reaction Hzco Hzo
How Buffers Help You Periodical 2015
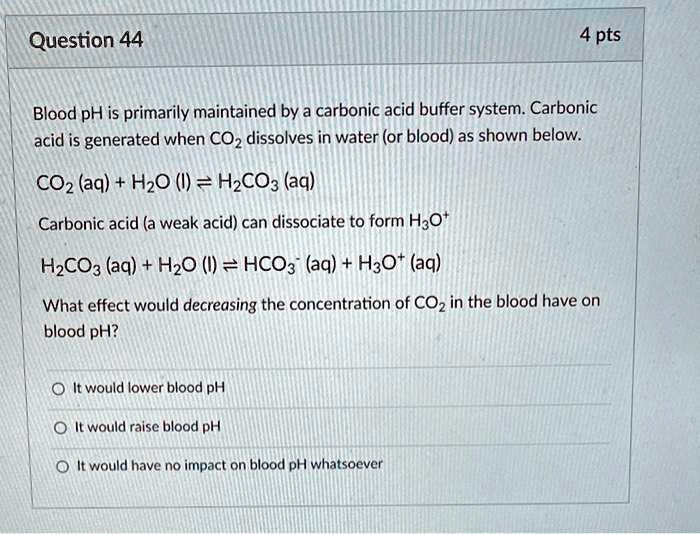
Solved Question 44 4 Pts Blood Ph Is Primarily Maintained By Carbonic Acid Buffer System Carbonic Acid Is Generated When Coz Dissolves In Water Or Blood As Shown Below Coz Aq Hzo

Mineralogy Of Carbonates Basic Geochemistry Geological Digressions

Chemical Buffer Systems And Acid Base Balance
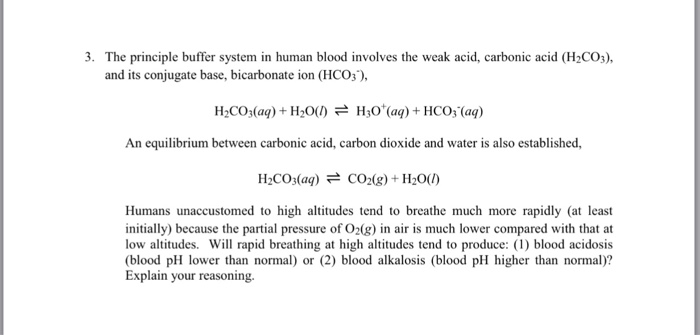
Solved The Principle Buffer System In Human Blood Involves Chegg Com
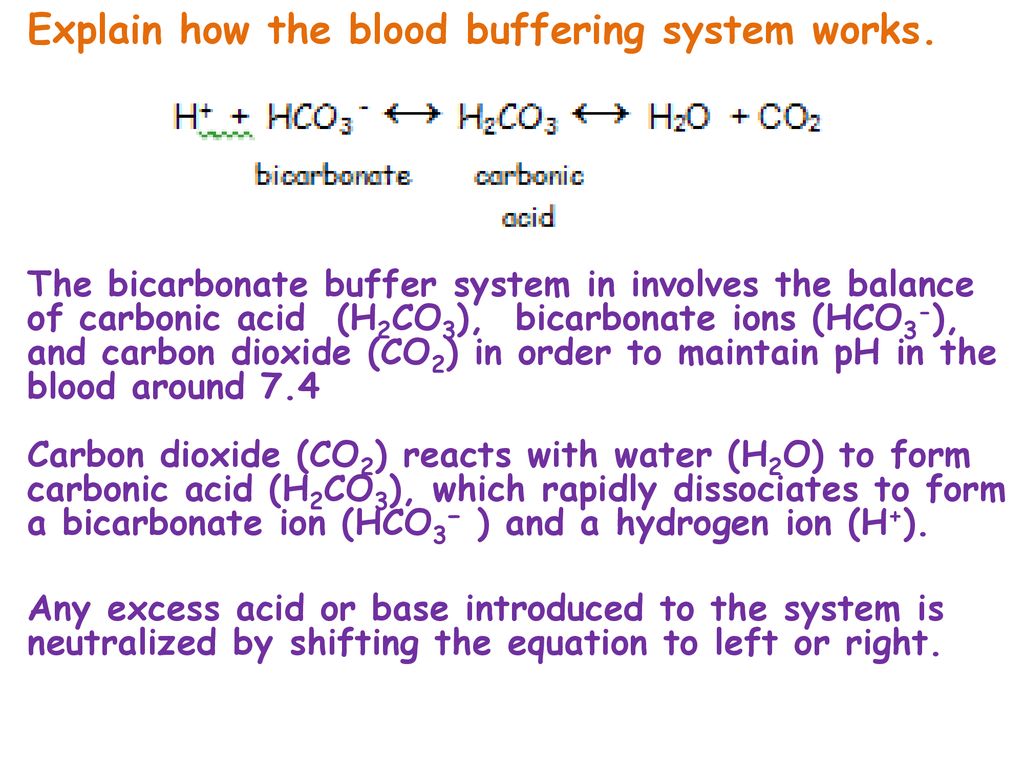
Earth Quakes And Ph Explain How The Blood Buffering System Works What Organs Are Involved How Do These Maintain The Ph Balance Of Blood Why Is Baking Ppt Download



Comments
Post a Comment